Spectral Distribution of Solar Radiation
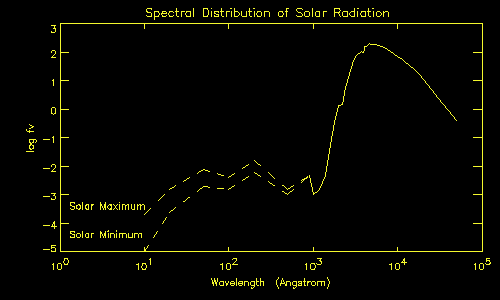
The graph below shows the amount of radiation produced by the Sun over the wavelength range 10 Angstroms (1 Angstrom = 10-10 metres; or 0.1 nanometres) to 50,000 Angstroms. This range spans from the extreme ultraviolet, through the ultraviolet, visible and into the infra-red. The vertical axis on the graph gives the amount of radiation received from the Sun above the Earth's atmosphere and so is not affected by the varying transmission of the atmosphere. The amount of radiation (fv) is expressed in units of erg/square cm/second/Angstrom and is plotted as a logarithm (log fv) because it varies by many powers of ten over the range in the graph.
The distribution of radiation can be divided into two regions. Above a wavelength of around 1000 Angstroms (solid curve) the radiation produced by the Sun is 'thermal' in origin - i.e. it arises because the Sun is a hot object. The spectral distribution has a shape known as a black body curve with the peak occurring at around 5000 Angstroms - in the middle of the range of wavelengths which we are able to see. For higher, or lower, wavelengths the radiation produced by the Sun decreases.
Within this range, the region from around 1000 Angstroms up to around 4000 Angstroms is the ultraviolet and this radiation is generally absorbed in the atmosphere of the Earth by the ozone layer. It is this radiation which, if not absorbed by the atmosphere, is important in the production of skin cancer. The amount of radiation reaching the ground depends on factors such as whether the Sun is close to overhead in the sky, and conditions in the atmosphere such as the concentration of ozone.
The second significant region in the figure is shown as two dashed lines below a wavelength of around 1000 Angstroms. There are two lines because, unlike the constant thermal radiation, this component varies with the solar cycle with a larger amount being produced by the Sun during the higher parts of the solar cycle than during the lower parts of the cycle. Normally, this component is absorbed by the atmosphere - in fact in the ionosphere which it is responsible for creating.
Material prepared by Richard Thompson





